- BioFlo PICC
- ABO RhD Blood Grouping Reagents
- AHG Reagents
- AMT
- Bone Graft
- Endotracheal Tube
- FibroScan® for liver disease
- Intercept Blood System
- Laparoscopic Range
- Nova Hepar P
- Phenotyping Reagents
- Smart Port + Titanium
- Smart Port Plastic
- Supplementary Range
- Surgical Stapler
- Wound & Pressure Ulcer
United Italian Trading (M) Sdn Bhd
607, Block C, No.9,
Jalan 16/11,
Phileo Damansara 1,
46350 Petaling Jaya,
Selangor, Malaysia.
FibroScan Expert 630
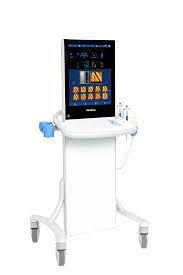
|
FibroScan Expert 630 Expand clinical capabilities
The new spleen stiffness measurement (SSM by VCTE™) and the embedded ultrasound localization system enable risk stratification of patients with advanced chronic liver disease and portal hypertension. SSM by VCTE™ is unique, patented and validated for portal hypertension assessment and can be used for risk stratification of patients with advanced chronic liver disease 4. It is a new marker for non-invasive evaluation of spleen stiffness. 109 peer-reviewed publications support the use of SSM by VCTE™. LSM by VCTE™ is unique, patented and validated for liver fibrosis assessment. It is the standard for non-invasive evaluation of liver stiffness1 with 3,245 international peer-reviewed publications.
CAP™ is unique patented and validated for liver steatosis assessment2,3 with 822 international peer-reviewed publications. 1. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237-264. doi:10.1016/j.jhep.2015.04.006. 2. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022-1030. doi:10.1016/j.jhep.2016.12.022. 3. Recio E, Cifuentes C, Macías J, et al. Interobserver concordance in controlled attenuation parameter measurement, a novel tool or the assessment of hepatic steatosis on the basis of transient elastography. Eur J Gastroenterol Hepatol. 2013;25(8):905-911.doi:10.1097/MEG.0b013e32835f4c3d. 4. Stefanescu H, Marasco G, Calès P, et al. A novel spleen-dedicated stiffness measurement by FibroScan® improves the screening of high-risk oesophageal varices. Liver Int. 2020;40(1):175-185. doi:10.1111/liv.14228. Product Videos
|
